How Is Temperature Related to the Motions of Molecules
At 100 C the mean molecular speed is about 17 faster than at 0 C. The speed of molecular movement.
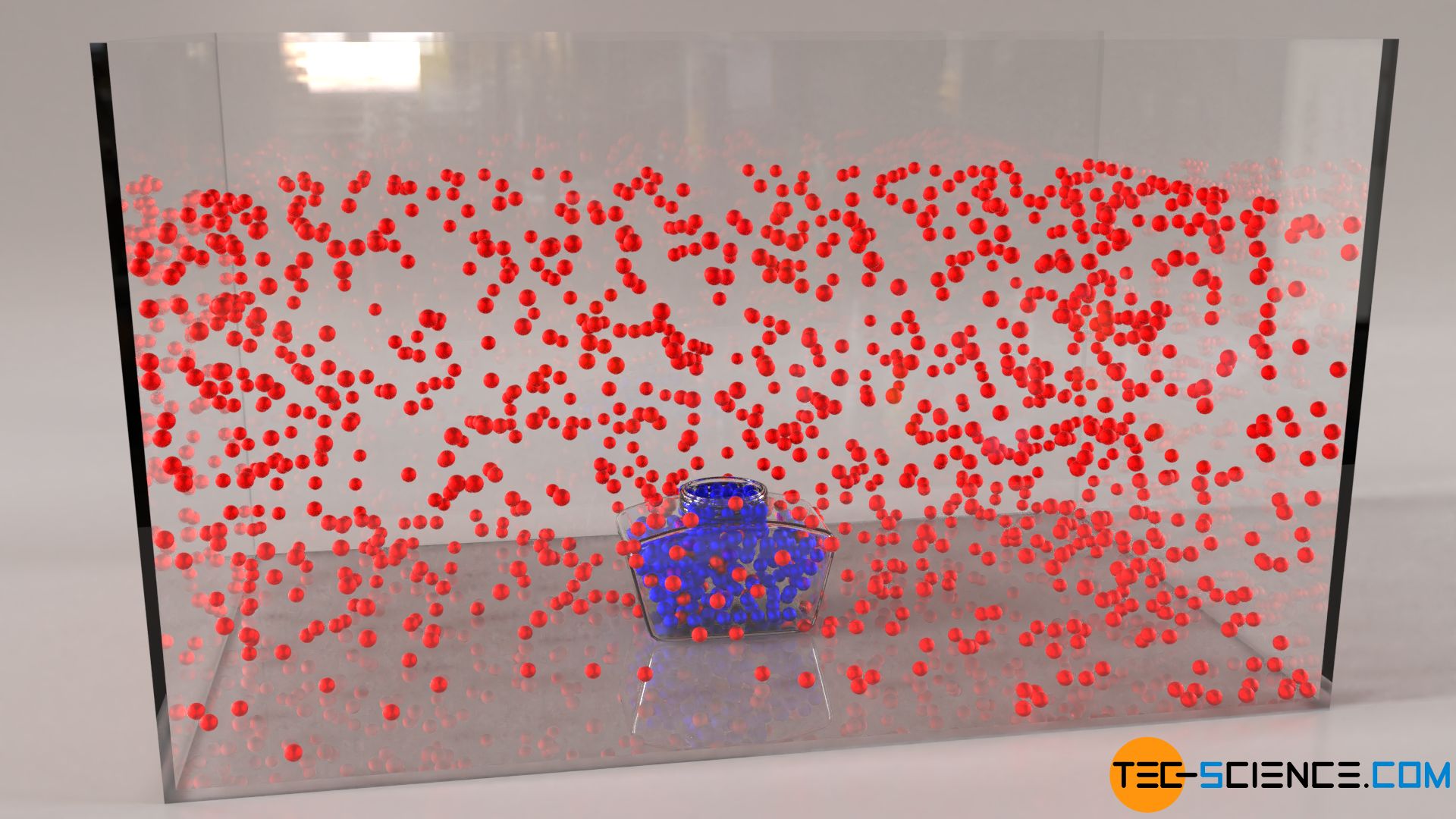
Temperature And Particle Motion Tec Science
Is random and erratic.
. For example Warm objects will have more molecular motion and the cold objects will have less molecular motion. This is because the temperature is the measurement of the average kinetic energy of the molecules and represents the motion of molecules. The higher the temperature the faster the molecules move.
Each unit on the Kelvin scale is equivalent to 1 C. Molecular motion is directly proportional to the temperature. Does the mean molecular speed change as much as the temperature as the water heats up.
If by some means the gas particles are accelerated to a very high speed in one direction KE certainly increased can we say the gas becomes hotter. A slight change in the Temperature raises the mean molecular speed of molecules. Its speed are part of the motion or molecules.
27315 K 0 C and 37315 K 100 C. As stated in the kinetic-molecular theory the temperature of a substance is related to the average kinetic energy of the particles of that substance. Because kinetic energy is the energy a substance has because of its molecules being in motion as a substance absorbs heat its molecules move faster thereby increasing the.
Check that the selected gas is. Increases with increasing temperature. The higher the temperature the faster the molecules move.
How is temperature related to the motions of molecules. When a substance is heated some of the absorbed energy is stored within the particles while some of the energy increases the motion of the particles. The temperature of a substance is a measure of the average kinetic energy of its particles kinetic energy is the energy of motion.
Similarly heat transfers energy among constituent molecules that increase the kinetic energy of molecules. All molecules are in constant motion. Which measures temperature from.
For each degree of temperature change the mean molecular speed increases by about 1 ms. So molecular motion is directly proportional to temperature. How is temperature related to the motions of molecules.
As the Temperature increases the mean molecular speed also increases. -as medium density goes up diffusion. Describe the motion of the hydrogen molecules.
What is the relationship between density of the medium and diffusion rate. The end result of increased molecular motion is that the object expands and takes up more space. The kinetic energy KE of a particle is equal to its mass times the square of its velocity divided by two.
In a similar way as the mixing of different gases or liquids can be attributed to Brownian motion a movement. Is lower in larger molecules. Heat temperature and the motion of molecules are all related.
Molecules of a liquid have more freedom of movement than those in a solid. When heat is added to a substance the molecules and atoms vibrate faster. Name 5 examples of Phase Changes Of Matter.
Heat is the total energy of the motion of the molecules inside the object or particle whereas Temperature is merely a measure of this energyThe relationship could be the more heated an object is there higher the. Heating water increases the kinetic energy of the molecules making them to move faster thus increasing the mean molecular speed of the molecules. As atoms vibrate faster the space between atoms increases.
Temperature affects the kinetic energy of molecules. Molecules in a gas have the greatest degree of motion. Temperature is a measure of the average kinetic energy of the molecules in a material.
The molecular motions are affected by heat and temperature. The motion and spacing of the particles determines the state of matter of the substance. The motion of molecules.
The temperature of a substance is directly related to its kinetic energy. Absolute zero the coldest possible temperature -27315 C. The temperature of a substance is directly related to its kinetic energy.
This increased motion increases the outward pressure of the gas an expected result. I n the kinetic theory of gasses increasing the temperature of a gas increases in average kinetic energy of the molecules causing increased motion. The higher the temperature the faster the diffusion will be because the stronger the molecule movement and thus the mixing.
KE mv2 2 A. Reflects the kinetic energy of molecules. On a microscopic scale thermal energy is related to the kinetic energy of molecules.
The higher the molecular motion the higher will be the temperature and vice-versa. How is temperature related to the motion of molecules. The temperature of a substance is directly related to its kinetic energyBecause kinetic energy is the energy a substance has because of its molecules being in motion as a substance absorbs heat its molecules move faster thereby increasing the substances kinetic.
How is temperature related to the motions of molecules. Based on the formula for kinetic energy how will the temperature change if you increase the average. Up to 24 cash back 2.
It is natural for regions containing greater molecular kinetic. Because kinetic energy is the energy a substance has because of its molecules being in motion as a substance absorbs heat its molecules move faster thereby increasing the substances kinetic energyJan 9 2020. Explain How The Temperature Of A Substance Is Related To The Kinetic Energy Of Its Molecules.
In ideal gas model temperature is the measure of average kinetic energy of the gas molecules. The greater a materials temperature the greater the thermal agitation of its constituent molecules manifested both in linear motion and vibrational modes. In the kinetic theory of gases random motion is assumed before deriving anything.

Ocean And Wind Currents Science For Kids Earth Science Meteorology

Boiling Point O Level Physics Quizzes O Level Physics Quiz 123 Questions And Answers Practice Physics Quizzes Based Questions Physics Quiz Physics O Levels

Weird Science Macroscopic Changes In Liquid Water Volume Manoa Hawaii Edu Exploringourfluidearth

First Law Of Thermodynamics Infographic Thermodynamics Chemistry Help Ap Physics
How Does Temperature Affect The Motion Of Particles In Solids Quora

Slac Builds One Of The World S Fastest Electron Cameras Electrons Machine Learning Methods Materials Science And Engineering

Chemistry Revision Notes Igcse Chemistry Revision Chemistry Notes Revision Notes

Sublimation Technology Knowing The Definition Of Dye Sublimation Printing Sublime Evaporation Science Classroom

Motion In A Plane Cbse Notes For Class 11 Physics Learn Cbse Class11physicsnotes Motioninaplanephysicsclass11 Physics Notes Physics Class

Rudra Education On Instagram In Today S Universe Particles Obey Certain Rules Most Of Them Have

Molecular Motion Energy And Temperature Youtube

General Chemistry Notes Full Course Pdf Notes Chemistrynotes Com Chemistry Notes Chemistry Basics Study Chemistry

Thermal Properties Of Matter Cbse Notes For Class 11 Physics Learn Cbse Class11physicsnotes Thermalpropertiesofma Physics Notes Physics Solar Constant
If Kinetic Energy Is Equal To 3 2kt And Kinetic Energy Is Related To Temperature Then How Comes Different Materials Have Different Heat Capacities I E If At A Given Change In Ke Energy
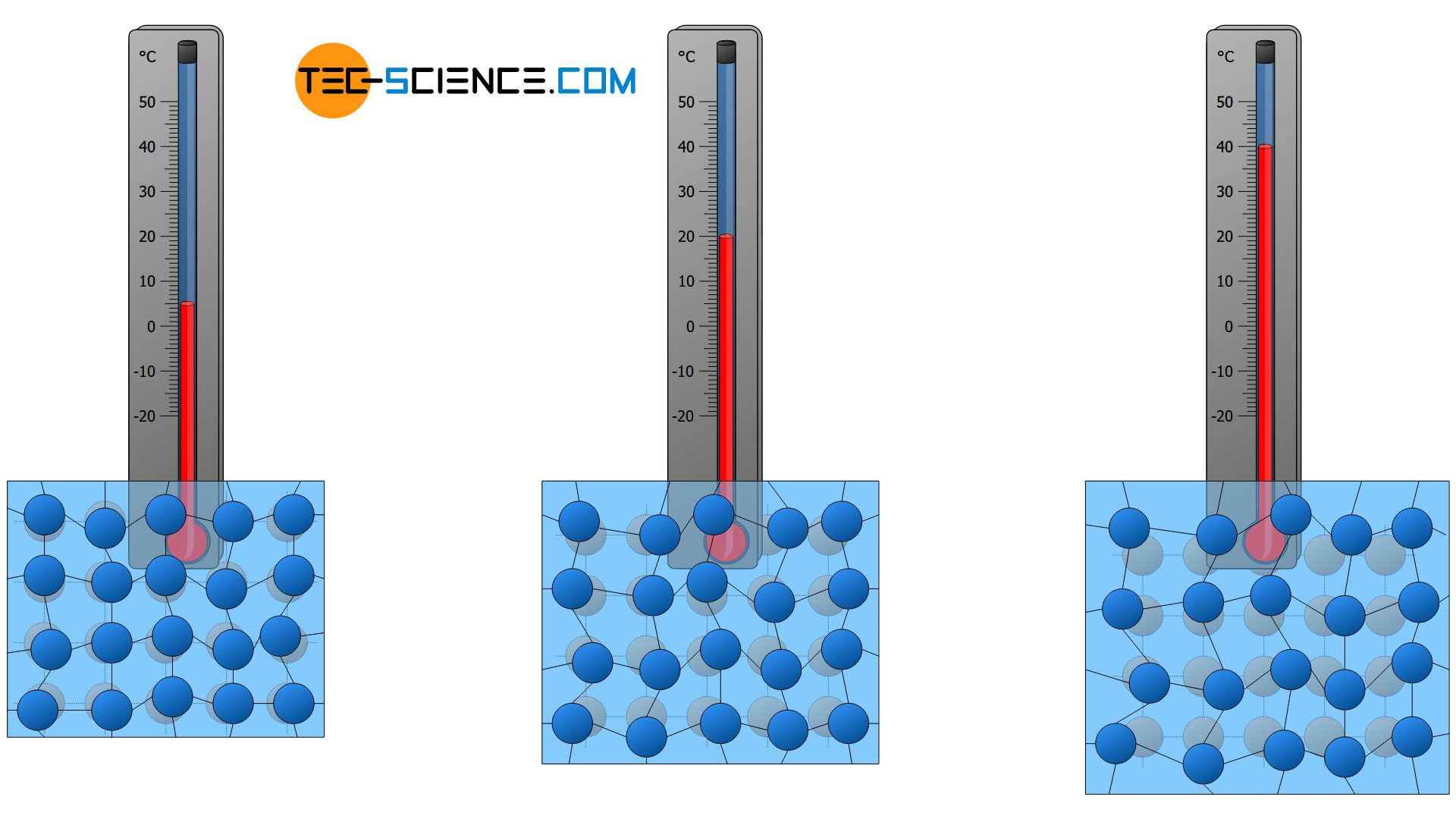
Temperature And Particle Motion Tec Science

52 Common Myths Rumors And Falsehoods Debunked Common Myths Myths Science

System Of Particles And Rotational Motion Cbse Notes For Class 11 Physics Learn Cbse Class11physicsnotes Systemofparticles Physics Notes Physics Class
Why Doesn T The Size Of A Molecule Increase With An Increase In Temperature Quora

Comments
Post a Comment